New Paper: fMRI study of working memory capacity in schizophrenia
/Hahn, B., Robinson, B. M., Leonard, C. J., Luck, S. J., & Gold, J. M. (2018). Posterior parietal cortex dysfunction is central to working memory storage and broad cognitive deficits in schizophrenia. The Journal of Neuroscience, 37, 8378–8387. https://doi.org/DOI: https://doi.org/10.1523/JNEUROSCI.0913-18.2018 https://doi.org/10.1523/JNEUROSCI.0913-18.2018.
In several behavioral studies using change detection/localization tasks, we have previously shown that people with schizophrenia (PSZ) exhibit large reductions in visual working memory storage capacity (Kmax). In one large study with 99 PSZ and 77 healthy control subjects (HCS), we found an effect size (Cohen's d) of 1.11, and the degree of Kmax reduction statistically accounted for approximately 40% of the reduction in overall cognitive ability exhibited by PSZ (as measured with the MATRICS Battery). Change detection tasks are much simpler than most working memory tasks, focus on storage rather than manipulation, and can be used across species. Thus, Kmax gives us a measure that is both neurobiologically tractable and strongly related to broad cognitive dysfunction.
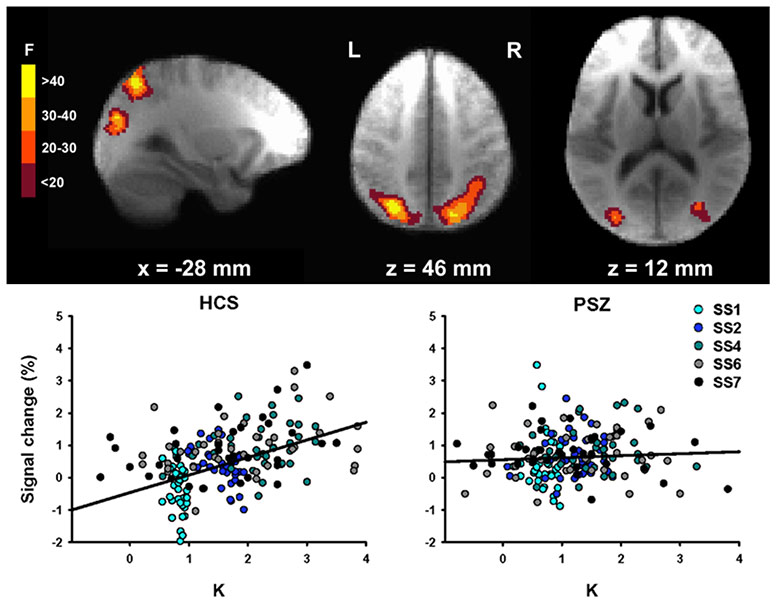
In our most recent work, led by Dr. Britta Hahn at the Maryland Psychiatric Research Center, we used fMRI to examine the neuroanatomical substrates of reduced Kmax in PSZ. We took advantage of an approach pioneered by Todd and Marois (2004, Nature), in which a whole-brain analysis is used to find clusters of voxels where the BOLD signal is related to the amount of information actually stored in working memory (K). As shown in the figure below, we found the same areas of posterior parietal cortex (PPC) that were observed by Todd and Marois.
In the left PPC, however, the K-dependent modulation of activity was reduced in PSZ relative to HCS. As shown in the scatterplots, the BOLD signal in this region was strongly related to the number of items being held in working memory (K) in HCS, but the function was essentially flat in PSZ. However, the overall level of activation was just as great in PSZ as in HCS (the Y intercept). The reduced slope was driven mainly by an overactivation in PSZ relative to HCS when relatively little information was being stored in memory. Moreover, the slope was strongly correlated with overall cognitive ability (again measured using the MATRICS Battery), and the degree of slope reduction statistically accounted for over 40% of the reduction in broad cognitive ability in PSZ.
One particularly interesting aspect of these results is that they point to posterior parietal cortex as a potential source of cognitive dysfunction in schizophrenia, whereas most research and theory has focused on prefrontal cortex. Studies with healthy young adults have consistently identified PPC as a major player in working memory capacity and in the ability to divide attention, both of which are strongly impaired in PSZ. We hope that our study motivates more research to examine the potential contribution of the PPC to cognitive dysfunction in schizophrenia.